Therapeutic Goods Administration Australia, #TGA
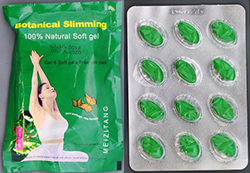
Meizitang Botanical Soft Gel Capsules
Meizitang Botanical Soft Gel Capsules pose a serious risk to your health and should not be taken
3 April 2019
The Therapeutic Goods Administration (TGA) has tested a product labelled Meizitang Botanical Soft Gel Capsules and found that:
- The capsules contain the undeclared substance Sildenafil & Sildenafil N-Oxide.
Consumers are advised that Sildenafil & Sildenafil N-Oxide are prescription-only substances in Australia.
The supply of Meizitang Botanical Soft Gel Capsules containing undisclosed Sildenafil & Sildenafil N-Oxide is illegal.
Meizitang Botanical Soft Gel Capsules have not been assessed by the TGA for quality, safety or efficacy as required under Australian legislation, and the place of manufacture is not approved by the TGA.
TGA investigations have shown that a number of people in Australia may have bought the product online.

Information for consumers
- Stop taking Meizitang Botanical Soft Gel Capsules and take any remaining capsules to your local pharmacy for safe disposal.
- If you have any concerns arising from your use of this product, consult your health care practitioner.
Action the TGA is taking
The TGA is working with the Australian Border Force (ABF) to help stop future shipments of Meizitang Botanical Soft Gel Capsules from entering Australia.
If these capsules are found at the border by the ABF they will be seized and destroyed.
The TGA is advising consumers to exercise extreme caution when purchasing medicines from unknown overseas internet sites and has produced a short video on the risks associated with buying medicines and medical devices online. Products purchased over the internet:
- may contain undisclosed and potentially harmful ingredients
- may not meet the same standards of quality, safety and efficacy as those approved by the TGA for supply in Australia.
Report counterfeit medicines and medical devices
If you are worried about counterfeit medicines or medical devices, and want to report an issue, you can report the matter to the TGA:
| Phone | 1800 020 653 |
|---|---|
| Online: | Report a perceived breach of the Therapeutic Goods Act or questionable practices relating to therapeutic products |
| info@tga.gov.au |
No comments:
Post a Comment